Beta Decay
Radioactivity is the spontaneous emission of radiation (energy) by certain unstable atomic nuclei.
This phenomenon, discovered by Henri Becquerel and Pierre & Marie Curie in 1896-1898, occurs in different forms.
Let's focus on beta decays, discovered a few years later by Ernest Rutherford.
There are two types of beta decays: beta minus and beta plus.
Both are described in more detail in the following picture gallery (Here: Help to understand Feynman diagrams):
-
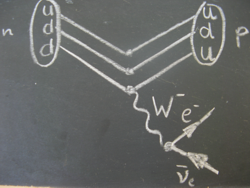 Beta minus decay is the transformation of an unstable nucleus accompanied by the emission of an electron and an electron anti-neutrino.
It happens when a neutron changes into a proton.
The electron and electron anti-neutrino come from the decay of the W-particle (here the W--particle).
Particle physicists see the decay like this: when one down quark of the proton transforms into an up quark, a W--particle is emitted.
The W--particle transforms into an electron and an electron anti-neutrino after the unbelievably short time of about 10-25 seconds.
The emitted electron is what is usually referred to as beta radiation.
Beta minus decay is the transformation of an unstable nucleus accompanied by the emission of an electron and an electron anti-neutrino.
It happens when a neutron changes into a proton.
The electron and electron anti-neutrino come from the decay of the W-particle (here the W--particle).
Particle physicists see the decay like this: when one down quark of the proton transforms into an up quark, a W--particle is emitted.
The W--particle transforms into an electron and an electron anti-neutrino after the unbelievably short time of about 10-25 seconds.
The emitted electron is what is usually referred to as beta radiation.
-
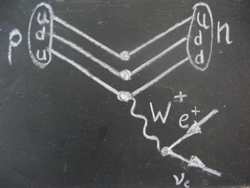 During beta plus decay, a positron is emitted from an unstable nucleus when a proton inside the nucleus transforms into a neutron.
An electron neutrino is also produced.
The positron and electron neutrino come from the decay of the W-particle (here the W+-particle).
Particle physicists see the decay like this: when one up quark of the proton transforms into a down quark, a W+-particle is emitted.
The W+-particle decays into a positron and an electron neutrino after the unbelievably short time of about 10-25 seconds.
The positron is what is generally referred to as beta radiation.
During beta plus decay, a positron is emitted from an unstable nucleus when a proton inside the nucleus transforms into a neutron.
An electron neutrino is also produced.
The positron and electron neutrino come from the decay of the W-particle (here the W+-particle).
Particle physicists see the decay like this: when one up quark of the proton transforms into a down quark, a W+-particle is emitted.
The W+-particle decays into a positron and an electron neutrino after the unbelievably short time of about 10-25 seconds.
The positron is what is generally referred to as beta radiation.
Conservation of electric charge
Have you noticed that the sum of electric charge remains the same during the beta decays?
Just calculate the electric charge before and after the decay of the existing particle.
Click
here to get back to the W-Path website.

